EQRS News Flash: New EQRS Clinical Reporting Fields: Parathyroid Hormone

August 2, 2022
The Centers for Medicare & Medicaid Services (CMS) has added new parathyroid hormone (PTH) reporting fields to the End Stage Renal Disease (ESRD) Quality Reporting System (EQRS). Dialysis facilities are now able to enter monthly PTH values on the Manage Clinical screen, under the Mineral Metabolism section in EQRS. The new PTH reporting fields include Parathyroid Hormone Value and date (Month, Day, Year), Parathyroid Hormone Method, and Parathyroid Hormone Upper Limit Assay Range (displayed in Figure 1 below).
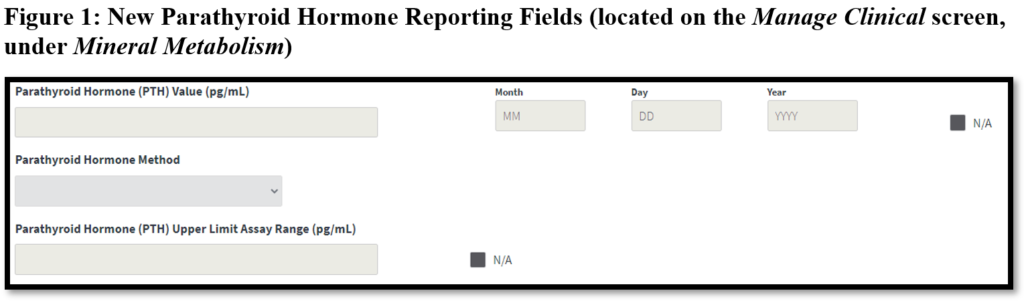
Facilities must select N/A if a PTH value was not measured for the patient for the clinical month. The Parathyroid Hormone Method, Parathyroid Upper Limit Assay Range and date fields are required when a PTH value is entered. Reporting PTH data in EQRS allows CMS to assess bone mineral management and adverse outcomes associated with the management of PTH among the ESRD patient population.
If your facility batch submits data or uses Health Information Exchange/Electronic Data Interchange methods for data submission, please follow the guidance provided by your organization.
For more information about the new PTH reporting fields in EQRS, please refer to the PTH Reporting Frequently Asked Questions resource that is available on the Education page on www.MyCROWNWeb.org.
If you are having technical issues with accessing the EQRS user interface, please contact the Center for Clinical Standards and Quality (CCSQ) Service Center. The CCSQ Service Center is open Monday-Friday 8 a.m. to 8 p.m. Eastern Time and can be reached via phone at: (866) 288-8912, via email at: qnetsupport-esrd@cms.hhs.gov, or by going to CCSQ Support Central.
