EQRS News Flash: New EQRS Reporting Process for Hepatitis B, Influenza and Pneumococcal Vaccinations

August 8, 2022
The Centers for Medicare & Medicaid Services (CMS) is scheduled to release a modified vaccination reporting process in the End Stage Renal Disease (ESRD) Quality Reporting System (EQRS) on *August 8, 2022. Beginning on this date, EQRS users will be required to enter any new vaccination data for Hepatitis B, Influenza, and Pneumococcal vaccinations on the Manage Patient screen under the new Vaccinations tab (displayed in Figure 1 below) in EQRS.
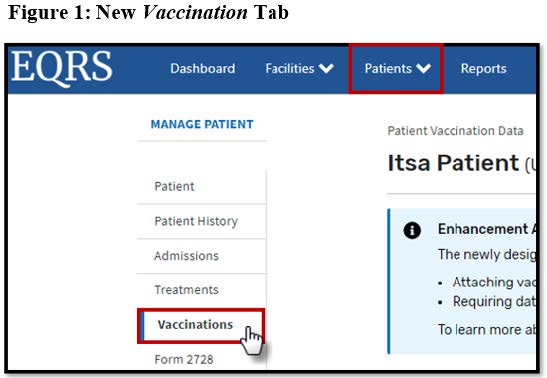
During the new Vaccination Module rollout, the most recent vaccination records for patients will be migrated from the Manage Clinical screen and will be visible on the new screens. On *October 1, 2022, the vaccination fields will no longer appear on the Manage Clinical screen.
Additionally, this new EQRS reporting process for vaccinations is an event-based reporting process. This means that EQRS users must submit vaccination data when a vaccination event occurs. A vaccination event occurs when a patient:
- Receives a vaccine dose administered by the reporting facility
- Receives a vaccine dose by an outside provider with documentation
- Receives a vaccine dose which is self-reported with or without documentation
- Does not receive a vaccine dose offered by the reporting facility
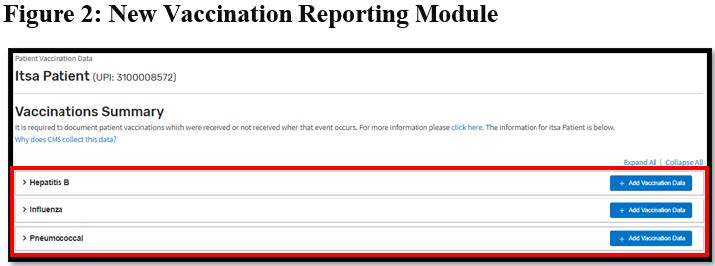
In addition to reporting vaccination information when a vaccination event occurs, facilities must also review patient vaccination history and enter any confirmed vaccination information in EQRS upon each patient admission to the facility. Facilities have until *October 1, 2022, to fully transition their Hepatitis B, Influenza and Pneumococcal vaccination reporting process in EQRS to the new Vaccinations Module (displayed in Figure 2 below) that is located under the Vaccinations tab on the Manage Patient screen.
If your facility batch submits data or uses Health Information Exchange/Electronic Data Interchange methods for data submission, please follow the guidance provided by your organization.
For more information about the new EQRS reporting process for vaccinations, please refer to the EQRS Vaccination Data Submission Requirements and Frequently Asked Questions resource that is available on the Education page on www.MyCROWNWeb.org.
If you are having technical issues with the EQRS user interface, please contact the Center for Clinical Standards and Quality (CCSQ) Service Center. The CCSQ Service Center is open Monday-Friday 8 a.m. to 8 p.m. Eastern Time and can be reached via phone at (866) 288-8912, email at qnetsupport-esrd@cms.hhs.gov, or by going to CCSQ Support Central. For general EQRS questions and questions about the ESRD Quality Incentive Program, please use the QualityNet Q&A Tool.
*The October 1, 2022, date listed in this announcement is subject to change.
