November 2022 EQRS News

In This Issue:
PY 2023 ESRD QIP Preview Period
Register for the EQRS Town Hall
How to Navigate the ESRD QIP UI
EQRS Data Submission Deadlines
Download the PDF.
View past Newsletters.
Upcoming Events:
New User Training
- November 15, 2022 • 2:00-3:30 PM ET
Web Resources:
Conditions for Coverage for ESRD Facilities
PY 2023 ESRD QIP Preview Period
The Payment Year (PY) 2023 End Stage Renal Disease Quality Incentive Program (ESRD QIP) preview period will open on November 14, 2022, and remain open for approximately 30 days, ending on December 14, 2022. During the PY 2023 ESRD QIP preview period, the Centers for Medicare & Medicaid Services (CMS) recommends the following activities:
- Facility or Corporate Points of Contact (POCs) and Viewers should review scores and reports via the ESRD QIP User Interface (UI) in the ESRD Quality Reporting System (EQRS)
- Facility and Corporate POCs should use the ESRD QIP UI in EQRS to submit any inquiries regarding PY 2023 scores before the preview period concludes
You can access reports based on your roles and availability. Reports include the Performance Score Report (PSR) and the Patient List Report (PLR).
PY 2023 ESRD QIP Preview Period supporting materials will be available from the QualityNet website in November 2022; these include the Guide to the PY 2023 ESRD QIP PSR and the PY 2023 ESRD QIP UI Quick Start Guide. Visit the QualityNet website to download the PY 2023 supporting materials by clicking on “Reports” or “Resources”.
Note: Per the calendar year (CY) 2023 ESRD QIP Prospective Payment System (PPS) final rule, there are seven paused clinical measures due to significant impacts from the COVID-19 public health emergency. The paused measures will not be used in PY 2023 ESRD QIP scoring or payment adjustments. However, measure rates will be publicly reported on CMS’ Care Compare website. These measures are: Standardized Hospitalization Ratio, Standardized Readmission Ratio, In-Center Hemodialysis Consumer Assessment of Healthcare Providers and Systems (ICH CAHPS), Long-Term Catheter Rate, Percentage of Prevalent Patients Waitlisted, Vascular Access Type: Standardized Fistula Rate, and Kt/V Dialysis Adequacy Comprehensive. As a result of these seven paused measures, the minimum Total Performance Score (TPS) a facility can receive to avoid a penalty in PY 2023 will be 83.For more information, refer to the CY 2023 ESRD PPS Final Rule, available on the Federal Register
Register for the EQRS Town Hall
Join the ESRD Outreach, Communication and Training (EOCT) Team for a Town Hall event for information on various EQRS reporting activities.
Date: Thursday, November 17, 2022
Time: 2:00 p.m. – 3:00 p.m. Eastern Time (ET)
The following topics will be discussed:
- EQRS Data Submission Deadlines
- EOCT Announcements
- ESRD QIP UI Training
- PY 2023 ESRD QIP Preview Period
How to Navigate the ESRD QIP UI
EQRS users must use EQRS to access the ESRD QIP interface (see Figure 1 below). Refer to the PY 2023 ESRD QIP UI Quick Start Guide for assistance in navigating and/or obtaining access to the QIP application within EQRS. The QIP application allows Facility Viewers, Facility POCs, Corporate Viewers, and POCs to perform the following tasks within the QIP application:
- View QIP performance scores and feedback
- Submit, save, and reply to preview period inquiries (Facility/Corporate POC only)
- View previously submitted preview period inquires
- View and download preview period reports
Access the ESRD QIP application though the EQRS login page at https://eqrs.cms.gov/globalapp/.
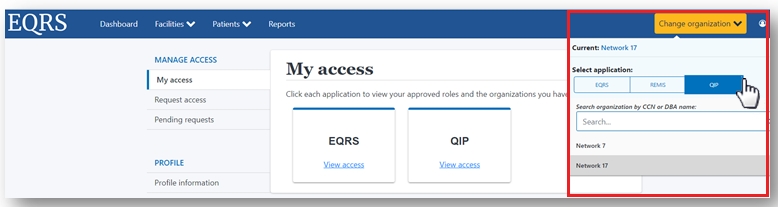
For more information about navigating the ESRD QIP UI, refer to the PY 2023 ESRD QIP User Interface Quick Start Guide resource on the Education page on www.MyCROWNWeb.org. You can also receive a tutorial on navigating the ESRD QIP UI and ESRD QIP Preview Period reports as part of the November 17, 2022, EQRS Town Hall event. Register for the November 17, 2022, EQRS Town Hall event here.
From the Chat Box
Question: Are dialysis facilities required to report monthly Parathyroid Hormone (PTH) values in EQRS?
Answer: No. Facilities are not required to report PTH data in EQRS. However, facilities must select N/A or “not applicable” for the PTH Value when a PTH value is not measured for the clinical month. If a PTH value is not entered or N/A is not selected, then you will receive a warning message when trying to submit monthly clinical data in EQRS. Additionally, if a PTH value is being reported for the month, then the other PTH reporting fields (including the date, PTH Method, and Upper Limit Assay Range) must be completed.
Question: Where can we find the value for the PTH Upper Limit Assay Range?
Answer: Although you can select the N/A (not applicable) checkbox for the PTH Upper Limit Assay Range, you should consult your organization and/or laboratory to confirm what value should be reported for the PTH Upper Limit Assay Range.
For more information about the EQRS PTH reporting fields, refer to the PTH Reporting Frequently Asked Questions resource available on the Education page on www.MyCROWNWeb.org.
How to Route Questions
Please do NOT include patients’ Protected Health Information (PHI) and Personally Identifiable Information (PII) (such as patient name, date of birth, social security number, Medicare Beneficiary Identifier, and Health Insurance Claim Number) when submitting a ticket and/or inquiry to the QualityNet Q&A Tool, CCSQ Service Center or ESRD Network. Any disclosure of PHI or PII should only be in accordance with, and to the extent permitted by, the Health Information Portability and Accountability Act (HIPAA), the HIPAA Privacy and Security Rules, and other applicable laws.
Please note: The EQRS identification number is the ONLY acceptable patient identifier when contacting the QualityNet Q&A Tool, CCSQ Service Center or ESRD Network.
| Question or Issue Type | Contact Information |
|
EQRS & ESRD QIP Questions:
|
QualityNet Question & Answer (Q&A) Tool: https://cmsqualitysupport. Note: To access EQRS training and/or educational materials, visit the Education page from MyCROWNWeb.org. |
EQRS System-related Questions or Issues:
|
The Center for Clinical Standards and Quality (CCSQ) Service Center: CCSQ Service Center hours of operation are Monday through Friday 8 a.m.-8p.m. ET. The CCSQ Service Center can be reached via: Phone: (866) 288-8912 Email: qnetsupport-esrd@cms.hhs.gov CCSQ Support Central (to create and track a ticket): https://cmsqualitysupport.servicenowservices.com/ccsq_support_central |
ESRD Network Assistance with:
|
ESRD Network Directory:
Contact your ESRD network directly. Use the link below if you need assistance finding your ESRD Network’s contact information. |
EQRS Data Submission Deadlines
Dialysis facilities must meet the EQRS data deadlines listed below to meet CMS reporting requirements. Failure to complete the submission of data by the deadlines listed in this announcement puts your facility at risk for an ESRD QIP payment reduction. The data submission deadline applies to all collection types (Hemodialysis and Peritoneal Dialysis) and to all submission methods. CMS strongly recommends that facilities complete large data submissions and audit batch submitted data prior to data submission deadlines.
| 2022 EQRS Data Submission Schedule for: EQRS Clinical Data |
|
| Reporting Month | Data Submission Deadline |
| September 2022 | November 30, 2022 at 11:59 PM PT |
| October 2022 | January 3, 2023 at 11:59 PM PT |
| November 2022 | January 31, 2023 at 11:59 PM PT |
| December 2022 | February 28, 2023 at 11:59 PM PT |
| 2022 EQRS Data Submission Schedule for: Clinical Depression Screening and Follow Up & ICH CAHPS Facility Attestation |
|
| Reporting Months | Data Submission Deadline |
| January 2022 – December 2022 (All months in 2021) |
February 28, 2023 at 11:59 PM PT |
The information included as part of this newsletter is current as of the date of release.

