June 2023 EQRS News

In This Issue:
CY 2024 ESRD PPS Proposed Rule
Register for the EOCT Town Hall
Care Compare Dialysis Data Release
ESRD QIP SHR, SRR, STrR Measure Updates
EQRS Data Submission Deadlines
Download the PDF.
View past Newsletters.
Upcoming Events:
New User Training
- July 18, 2023 • 2:00-3:30 PM ET
Web Resources:
Conditions for Coverage for ESRD Facilities
CY 2024 ESRD PPS Proposed Rule
On June 26, 2023, the Calendar Year (CY) 2024 End-Stage Renal Disease (ESRD) Prospective Payment System (PPS) Proposed Rule was displayed on the Federal Register website. Each year, the Centers for Medicare & Medicaid Services (CMS) issues a proposed rule to update Medicare payment policies and rates under the PPS. This rule proposes updates to the ESRD Quality Incentive Program (ESRD QIP) requirements. In addition, CMS is proposing policies that reflect commitment to achieving equity in healthcare. CMS requests public comment on the proposals presented in the proposed rule. To submit a public comment, follow the instructions in the proposed rule that is published on the Federal Register website.
For more information, click on the following links:
Register for the EOCT Town Hall
Join the ESRD Outreach, Communication, and Training (EOCT) Team for a Town Hall event for information on various ESRD reporting activities, new ESRD Quality Reporting System (EQRS) features including the Peritonitis Infections Module and the Transplant Dashboard for dialysis facilities, and updates to the ESRD QIP Standardized Hospitalization Ratio (SHR), Standardized Readmission Ratio (SRR), Standardized Transfusion Ratio (STrR) measures, and more.
Date: June 29, 2023
Time: 2:00 p.m. – 3:30 p.m. ET
The following topics will be discussed:
- ESRD Data Submission Deadlines and EOCT Announcements
- New EQRS Features: Peritonitis Infections Module and Transplant Dashboard for Dialysis Facilities
- ESRD QIP SHR, SRR and STrR Measure Updates
- Frequently Asked Questions
Care Compare Dialysis Data Release on Medicare.gov
In April 2023, CMS released new dialysis facility data on Care Compare on the Medicare.gov website. Through this website, patients and healthcare stakeholders can view and compare quality data about dialysis facilities. These data are organized into a series of measures from which CMS creates a star rating system that helps ensure safety, quality, and transparency among dialysis facilities. The star rating system also helps patients make educated decisions about where to get their dialysis treatments. To access the latest dialysis star ratings on Care Compare on the Medicare.gov website, go to: https://www.medicare.gov/care-compare/.
Additionally, CMS also released the Payment Year (PY) 2023 ESRD QIP public reporting files in April 2023. These reporting files contain scoring and measure performance for the ESRD QIP measures for all eligible outpatient dialysis facilities in PY 2023. The public reporting files promote data transparency and, like the star rating system, are intended to help patients make informed decisions when selecting their dialysis facility. The PY 2023 ESRD QIP public reporting files are available on the CMS.gov Provider Data Catalog website for the following ESRD QIP domain topics and quality reporting measures:
Patient & Family Engagement
- In-Center Hemodialysis Consumer Assessment of Healthcare Providers and Systems (ICH CAHPS): Individual measures and star rating of ICH CAHPS survey of patients’ experiences (i.e., kidney doctor’s communication and caring; dialysis center staff care and operations; providing information to patients; rating of kidney doctors, the dialysis center staff; the dialysis facility and overall star rating of ICH CAHPS survey of patients’ experiences)
Care Coordination
- Standardized Readmission Ratio (SRR)
- Standardized Hospitalization Ratio (SHR)
- Percentage of Prevalent Patients Waitlisted (PPPW)
- Clinical Depression Screening and Follow-Up
Clinical Care
- Kt/V Dialysis Adequacy (comprehensive)
- Vascular Access:
- Standardized Fistula Rate (SFR)
- Long-term Catheter Rate
- Standardized Transfusion Ratio (STrR)
- Hypercalcemia
- Ultrafiltration Rate (UFR)
Safety
- National Healthcare Safety Network (NHSN) Bloodstream Infection (BSI) in Hemodialysis Patients
- NHSN Dialysis Event Reporting
- Medication Reconciliation (MedRec)
Medicare beneficiaries, facilities, and other stakeholders can find this information on Care Compare on the Medicare.gov website, as well as the Public Reporting & Certificates page on the ESRD QIP section of CMS.gov.
From the Chat Box
Question: I need to modify a form that has been submitted in EQRS. Who can help me with this?
Answer: Contact your ESRD Network for assistance with modifying a CMS-2728 or CMS-2746 Form that has been submitted in EQRS, if any of the following criteria are met:
- An edit request is made within 60 days from the form’s submission date in EQRS. Please note that changes that impact patient coverage such as date of first dialysis have no time limit for making changes.
- The request is coming from the facility that originally submitted the form. Only the submitting facility can request to make a change to that form.
- Edits to the patient’s demographics screen and/or the admit/treatment information are completed prior to submitting a form change request to the ESRD Network
- If the edit request is for a form that is version 2018 or newer:
Click this link to find your ESRD Network contact information: https://esrdncc.org/en/ESRD-network-map/.
Contact the Center for Clinical Standards and Quality (CCSQ) Service Center for assistance with modifying a CMS-2728 Form that has been submitted in EQRS, if any of the following criteria are met:
- The edit request involves:
- Deleting a form that has been submitted in EQRS.
- Changing a physician name on a form that has been submitted in EQRS.
- Changing signature dates on a form that has been submitted in EQRS.
- The edit request is for a form that is version 2014 or older.
The CCSQ Service Center can be reached via phone at (866) 288-8912, email at qnetsupport-esrd@cms.hhs.gov, or by going to CCSQ Support Central.
Please note: Any changes made to a previously submitted form will change the submission date in EQRS and in turn may negatively impact facility compliance of the form.
ESRD QIP SHR, SRR, and STrR Measure Updates
Beginning in PY 2024, CMS is updating the ESRD QIP technical measure specifications to express SHR and SRR clinical measure results as rates. To align with this technical update, CMS will also be converting the STrR reporting measure to a clinical measure and expressing the STrR measure as a rate beginning in PY 2025. Changing the SHR, SRR and STrR measure results from ratios to rates helps providers and patients better understand a facility’s performance on the measures and makes it easier for facilities to track their performance from year to year. Converting the STrR reporting measure to a clinical measure requires CMS to update the scoring methodology for the STrR measure so that facilities that meet previously finalized minimum data and eligibility requirements would receive a score on the STrR clinical measure based on the actual clinical values reported by the facility, rather than the completeness of the reported data. Table 1 below provides a summary of these measure changes.
Table1: ESRD QIP SHR, SRR, STrR Measure Updates
| ESRD QIP Measure | Payment Year | Measure Type | Updated Measure Description |
| SHR | Beginning in PY 2024 | Clinical |
|
| SRR | Beginning in PY 2024 | Clinical |
|
| STrR | Beginning in PY 2025 | Change from Reporting to Clinical |
|
For additional information on the SHR, SRR and STrR measures, refer to the following resources:
- CY 2023 ESRD PPS Final Rule available in the Federal Register website
- ESRD QIP CY 2023 Technical Measure Specifications document
New Features in EQRS
Peritonitis Infections Module: On June 26, 2023, CMS released a Peritonitis Infections Module in EQRS. This new module captures peritonitis infection data and rates in peritoneal dialysis patients. Dialysis facilities are required to enter peritonitis infection data on the Manage Patient screen under the new Infections tab in EQRS (Figure 1). The new EQRS Peritonitis Infections Module reporting process requires dialysis facilities to document peritoneal dialysis infections when they occur. EQRS users can enter data for the following reporting sections within the module:
- Patient and Event Details
- Risk Factors
- Lab and Diagnostic Testing
- Signs and Symptoms
- Outcomes
Please note: If your facility batch submits data or uses Health Information Exchange/Electronic Data Interchange methods for data submission, please follow the guidance provided by your organization.
Figure 1: Peritonitis Infections Module
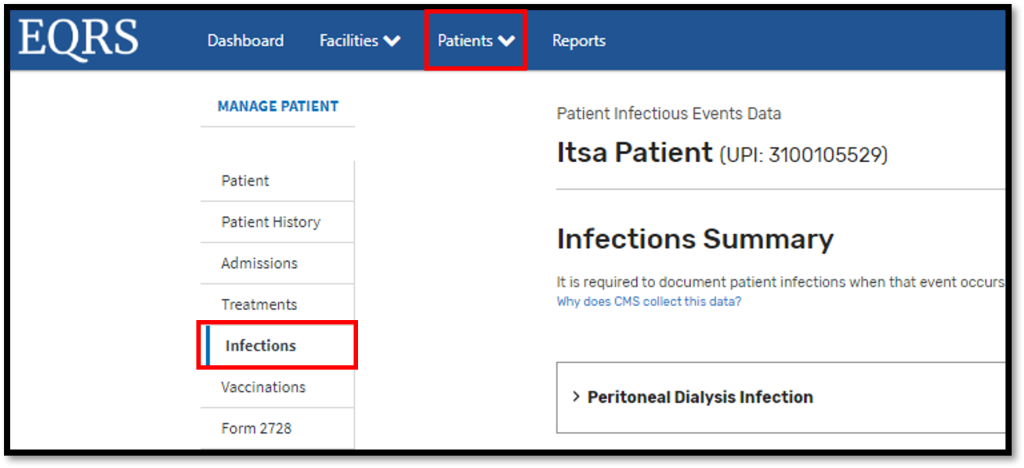
For more information about the new Peritonitis Infections Module, please refer to the CMS Infection Event Data Submission Requirements resource that is available on the Education page on www.MyCROWNWeb.org. Additionally, the Peritonitis Infections Module will be presented on the June 29, 2023, EOCT Town Hall event. Register for the June Town Hall event here.
Transplant Dashboard for Dialysis Facilities: On May 17, 2023, CMS released a Transplant Dashboard for dialysis facilities in EQRS (Figure 2). The Transplant Dashboard lists EQRS and United Network for Organ Sharing information for all living patients in a facility who are listed with one or more transplant centers and includes the contact information for those transplant centers. Dialysis facilities can access the Transplant Dashboard by clicking on the Dialysis Facility Transplant Waitlist link at the bottom of the Dashboard screen in EQRS. Additionally, facilities can view dashboard information for the purpose of:
- Tracking a patient’s waitlist status
- Communicating with transplant centers where their patients are listed
- Helping patients maintain their active transplant status to successfully receive a kidney transplant
Figure 2: Transplant Dashboard for Dialysis Facilities
How to Route Questions
Please do NOT include patients’ Protected Health Information (PHI) and Personally Identifiable Information (PII) (such as patient name, date of birth, social security number, Medicare Beneficiary Identifier, and Health Insurance Claim Number) when submitting a ticket and/or inquiry to the QualityNet Q&A Tool, CCSQ Service Center or ESRD Network. Any disclosure of PHI or PII should only be in accordance with, and to the extent permitted by, the Health Information Portability and Accountability Act (HIPAA), the HIPAA Privacy and Security Rules, and other applicable laws.
Please note: The EQRS identification number is the ONLY acceptable patient identifier when contacting the QualityNet Q&A Tool, CCSQ Service Center, or ESRD Network.
The table below contains contact information organized by question type:
| Question or Issue Type | Contact Information |
|
EQRS & ESRD QIP Questions:
|
QualityNet Question & Answer (Q&A) Tool: https://cmsqualitysupport. Note: Access EQRS training and/or educational materials on the Education page on MyCROWNWeb.org. |
EQRS System-related Questions or Issues:
|
The Center for Clinical Standards and Quality (CCSQ) Service Center can be reached Monday-Friday 8 a.m.-8p.m. ET. via: Phone: (866) 288-8912 Email: qnetsupport-esrd@cms.hhs.gov CCSQ Support Central: https://cmsqualitysupport.servicenowservices.com/ccsq_support_central |
ESRD Network Assistance with:
|
ESRD Network Directory:
Contact your ESRD Network directly. Use the link below if you need assistance finding your ESRD Network’s contact information. |
EQRS Data Submission Deadlines
Dialysis facilities must meet the EQRS data deadlines listed below to meet CMS reporting requirements. Please note, if the last day of the month falls on a Friday, Saturday, or Sunday, or on a federal holiday, the deadline will occur on the next federal business day. Not meeting the required reporting deadlines listed in this announcement puts your facility at risk for an ESRD QIP payment reduction. The data submission deadline applies to all collection types (Hemodialysis and Peritoneal Dialysis) and to all submission methods. CMS strongly recommends that facilities complete large data submissions and audit batch submitted data prior to data submission deadlines.
| 2023 EQRS Submission Schedule for: EQRS Clinical Data |
|
| Reporting Month | Data Submission Deadline |
| April 2023 | July 3, 2023, at 11:59 PM Pacific Time (PT) |
| May 2023 | July 31, 2023, at 11:59 PM PT |
| June 2023 | August 31, 2023, at 11:59 PM PT |
| July 2023 | October 2, 2023, at 11:59 PM PT |
| August 2023 | October 31, 2023, at 11:59 PM PT |
| September 2023 | November 30, 2023, at 11:59 PM PT |
| October 2023 | January 2, 2024, at 11:59 PM PT |
| November 2023 | January 31, 2024, at 11:59 PM PT |
| December 2023 | February 29, 2024, at 11:59 PM PT |
| 2023 EQRS Submission Schedule for: Depression Screening and Follow Up Assessments |
|
| Assessment Period | Data Submission Deadline |
| January 2023 – December 2023 (All months in 2023) |
February 29, 2024, at 11:59 PM PT |
| 2023 EQRS Submission Schedule for: In-Center Hemodialysis Consumer Assessment of Healthcare Providers Systems (ICH CAHPS) Facility Attestation |
|
| Attestation Period | Submission Deadline |
| January 2023 – December 2023 (All months in 2023) |
February 29, 2024, at 11:59 PM PT |
The information included as part of this newsletter is current as of the date of release.

