March 2024 EQRS News

In This Issue:
CMS-2744 ESRD Annual Facility Survey
Register for the March EOCT Town Hall
Care Compare on Medicare.gov Data Update
New MedRec Reporting Field in EQRS
ESRD Data Submission Deadlines
Download the PDF.
View past Newsletters.
Upcoming Events:
New User Training
- March 19, 2024 • 2:00-3:30 PM ET
Web Resources:
Conditions for Coverage for ESRD Facilities
CMS-2744 ESRD Annual Facility Survey
Each calendar year (CY), the Centers for Medicare & Medicaid Services (CMS) requires all Medicare-certified dialysis and transplant facilities to complete the CMS-2744 survey in the End Stage Renal Disease (ESRD) Quality Reporting System (EQRS). This survey provides a final count of all patients and treatments provided by a dialysis or transplant facility during the prior CY. It also includes important facility and patient demographic information, such as Medicare status, vocational rehabilitation, and facility staff counts.
Facilities can begin the CMS-2744 survey completion process by generating a CMS-2744 survey in EQRS. Before submitting the survey to the ESRD Network, facilities must review all survey information for accuracy and correct any identified errors. To verify auto-populated data on the survey, dialysis facilities can download four reports and transplant facilities can download one report from the View Facility Form 2744 screen (Figure 1a (dialysis facilities) and Figure 1b (transplant facilities)) in EQRS. Facilities should review these reports to identify any errors and make corrections, as needed. EQRS users must regenerate the survey to reflect any survey updates. Lastly, once a facility submits the completed survey to their ESRD Network for review, they must monitor the status of their form(s) in EQRS and work with their ESRD Network to finalize the form(s).
Please note: Please follow the guidance provided by your organization, as some organizations complete their surveys at the corporate level. Additionally, facilities are required to submit the completed survey by the deadline established by their ESRD Network. Use this link to find the contact information for your local ESRD Network: https://esrdncc.org/en/ESRD-network-map/.
Figure 1a: Downloadable reports on the View Facility Form 2744 for dialysis facilities
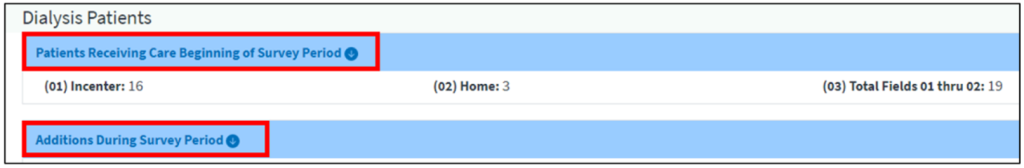
Figure 1b: Downloadable on the View Facility Form 2744 for Transplant Facilities
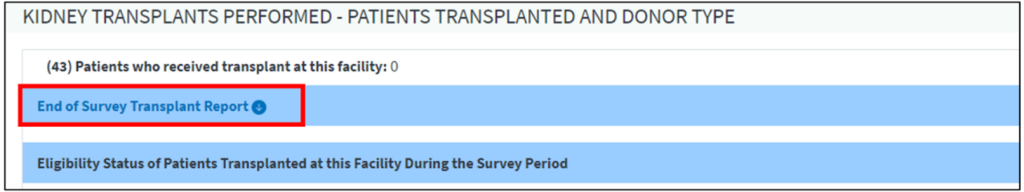
For additional information on how to complete the CMS-2744 survey, refer to the 2023 CMS-2744 Training Aid that is available on the Education page on www.MyCROWNWeb.org.
Register for the March EOCT Town Hall
Join the ESRD Outreach, Communication, and Training (EOCT) Team for a Town Hall event on ESRD reporting activities and deadlines, the CMS-2744 Annual Facility Survey, EQRS updates, including recently added features.
Date: March 14, 2024
Time: 2:00 p.m. – 3:30 p.m. Eastern Time
Presenter will discuss the following topics:
- ESRD Data Submission Deadlines
- EQRS updates including the new Facility Influenza Dashboard Reports and the new Medication Reconciliation (MedRec) reporting field
- CMS-2744 Annual Facility Survey
- Frequently Asked Questions
Care Compare on Medicare.gov Data Update
In January 2024, CMS released new dialysis facility data on Care Compare on Medicare.gov (Medicare.gov/care-compare); the January 2024 refresh is one of a series of updates made each year on the Medicare.gov/care-compare website. Through this website, patients and facilities can view and compare quality data about dialysis facilities. CMS uses these data, organized into a series of measures, to create a star rating system that can help patients make educated decisions about where to get their dialysis treatments.
Additionally, CMS also released the payment year (PY) 2024 ESRD Quality Incentive Program (QIP) public reporting files. These files contain scoring and measure performance for ESRD QIP measures, as well as any associated payment reductions, for all eligible outpatient dialysis facilities in PY 2024. The PY 2024 ESRD QIP public reporting files are available on the CMS.gov Provider Data Catalog website for the following ESRD QIP measures:
Care Coordination
- Standardized Readmission Ratio (SRR)
- Standardized Hospitalization Ratio (SHR)
- Percentage of Prevalent Patients Waitlisted (PPPW)
Clinical Care
- Kt/V Dialysis Adequacy (comprehensive)
- Vascular Access Type:
- Standardized Fistula Rate (SFR)
- Long-term Catheter Rate
- Standardized Transfusion Ratio (STrR)
Safety
- National Healthcare Safety Network (NHSN) Bloodstream Infection (BSI) in Hemodialysis Patients
Reporting
- Clinical Depression Screening and Follow-Up
- Hypercalcemia
- Ultrafiltration Rate (UFR)
- NHSN Dialysis Event Reporting
- Medication Reconciliation (MedRec)
- COVID-19 Vaccination Coverage Among Healthcare Personnel (HCP)
Medicare beneficiaries, facilities, stakeholders, and interested members of the general public can find this information on the Care Compare website on Medicare.gov (Medicare.gov/care-compare), as well as the Public Reporting & Certificates page on the ESRD QIP section of the CMS.gov website.
New Dashboard Reports: Facility Pneumococcal Pneumonia Vaccination and Facility Influenza Vaccination
On September 5, 2023, CMS added a Dashboard Reports feature in EQRS to allow users to generate various EQRS reports from the available Dashboards. Users can access this feature by clicking Reports Dashboards from the Reports drop-down tab in EQRS (Figure 2). The two most recently added dashboards are the Facility Pneumococcal Pneumonia Vaccination Dashboard and Facility Influenza Vaccination Dashboard.
The Facility Pneumococcal Pneumonia Vaccination Dashboard contains two reports: the Facility Summary report and Patient Details report. The Facility Summary report reflects facility-level pneumococcal pneumonia vaccination information in EQRS. This includes the number of patients who are up to date on their vaccines, the number of patients who are fully vaccinated, the number of patients who are eligible for a vaccination(s), and the pneumococcal pneumonia vaccination rate in the facility. The Patient Details report reflects patient-level pneumococcal pneumonia vaccination information in EQRS. This includes patient vaccination statuses and vaccination dates for the pneumococcal conjugate vaccine (PCV)20, PCV15, PCV13, and the pneumococcal polysaccharide vaccine (PPSV)23.
The Facility Influenza Vaccination Dashboard contains two reports: the Facility Summary report and Patient Details report. The Facility Summary report reflects facility-level influenza vaccination information in EQRS. This includes the number of patients who are up to date on their flu vaccine, the number of patients who are eligible for a flu vaccination, and the flu vaccination rate in the facility. The Patient Details report reflects patient-level flu vaccination information in EQRS. This includes patient vaccination statuses, vaccination dates, and vaccine type, for the current influenza season (August 1st– April 30th).
As a reminder, users can download and print each report using the Export to CSV or Export to Excel function within the dashboard. Please note that the CSV is the preferred download method for large files, and dashboard content is dictated by the user’s access and role in EQRS. CMS will continue to add new dashboards to the Reports screen in EQRS and will notify the ESRD community via email about new dashboard releases in EQRS.
Figure 2: Accessing Reports Dashboards in EQRS
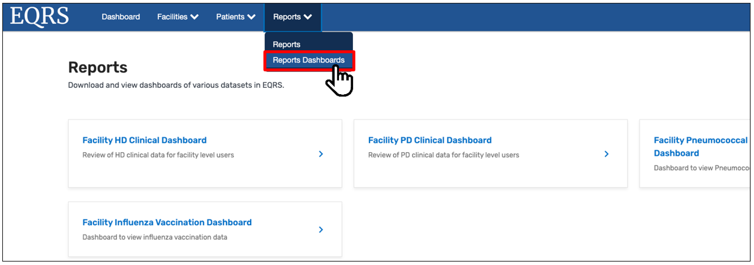
For additional information on the Facility Pneumococcal Pneumonia Vaccination Dashboard, please refer to the December Town Hall slides and recording, available on the Events page on www.MyCROWNWeb.org. For additional information on the Facility Influenza Vaccination Dashboard, please register for the upcoming March 14, 2024, EOCT Town Hall event. Register for the March Town Hall event here.
From the Chat Box
Question: Where can I go to find information on the ESRD QIP and guidelines on how to improve our ESRD QIP scores?
Answer: For general information on ESRD QIP reporting activities and how to report data for the ESRD QIP, please refer to the ESRD QIP Successful Reporting Guide available on the Education page on www.MyCROWNWeb.org. This guide provides information on submitting ESRD QIP data, meeting ESRD QIP data deadlines, accessing ESRD QIP reports and resources, and more! The ESRD QIP Successful Reporting Guide will be updated and posted annually to reflect the most current reporting period information; the current guide highlights the ESRD QIP measures for the CY 2024 reporting period for PY 2026.
Other helpful resources include the ESRD QIP Technical Measures Specifications and ESRD Measures Manual available on the CMS.gov website. The CY 2024 ESRD QIP Technical Measures Specifications provides detailed information on ESRD QIP measures (including measure descriptions, measure type, numerator and denominator statements, exclusion criteria, minimum data requirements, data sources, and additional information). The ESRD Measures Manual for the 2024 Performance Period provides detailed descriptions of how ESRD measure scores are calculated and how CMS evaluates the quality of dialysis care (numerator and denominator calculations and definitions, facility and patient level exclusions, methods used to determine exclusion criteria and calculate intermediary variables, facility mapping, changes in ownership, etc.).
For assistance with general ESRD QIP related questions, please contact the ESRD QIP Team via the QualityNet Q&A Tool.
New Medication Reconciliation Reporting Field
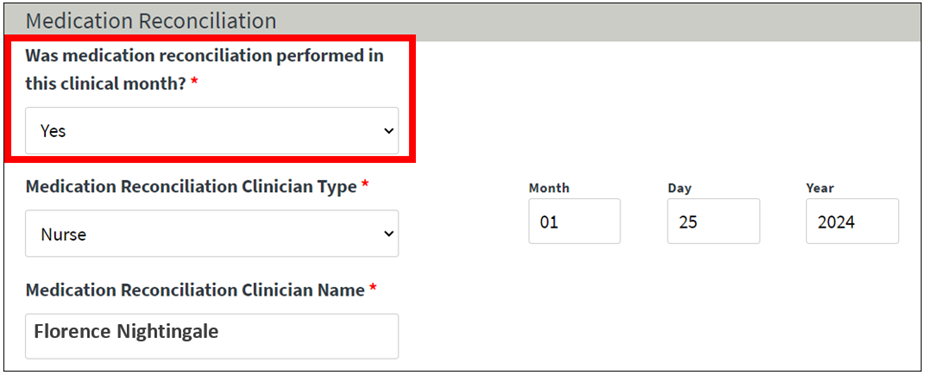
On January 1, 2024, CMS added a new Medication Reconciliation (MedRec) reporting field on the Manage Clinical screen in EQRS (Figure 3). Starting with the January 2024 clinical month, dialysis facilities are required to complete the new MedRec field under the Medication Reconciliation section of the Manage Clinical screen in EQRS, for every clinical month. Users must select “Yes” or “No” from the new drop-down field (Was medication reconciliation performed in this clinical month?), to complete reporting for the new field. If “Yes” is selected, the remaining fields in the section are required; if “No” is selected the remaining fields are grayed out and will not accept data. All fields in the Medication Reconciliation section must be completed (as indicated in Figure 3) to receive ESRD QIP credit for this measure. For additional information on MedRec reporting in EQRS, please register for the upcoming March 14, 2024, EOCT Town Hall event. Register for the March Town Hall event here.
Please note: If your facility batch submits or uses Health Information Exchange/Electronic Data Interchange methods for data submission, please follow the guidance provided by your organization.
Figure 3: New MedRec Reporting Field in EQRS
How to Route Questions
Please do NOT include patients’ Protected Health Information (PHI) and Personally Identifiable Information (PII) (such as patient name, date of birth, social security number, Medicare Beneficiary Identifier, and Health Insurance Claim Number) when submitting a ticket and/or inquiry to the QualityNet Q&A Tool, CCSQ Service Center, or ESRD Network. Any disclosure of PHI or PII should only be in accordance with, and to the extent permitted by, the Health Information Portability and Accountability Act (HIPAA), the HIPAA Privacy and Security Rules, and other applicable laws.
Please note: The EQRS identification number is the ONLY acceptable patient identifier when contacting the QualityNet Q&A Tool, CCSQ Service Center or ESRD Network.
The table below contains contact information organized by question type:
| Question or Issue Type | Contact Information |
|
EQRS & ESRD QIP Questions:
|
QualityNet Question & Answer (Q&A) Tool Note: Access EQRS training and/or educational materials on the Education page on MyCROWNWeb.org. |
EQRS System-related Questions or Issues:
|
The Center for Clinical Standards and Quality (CCSQ) Service Center can be reached Monday-Friday 8 a.m.-8p.m. ET via:
|
ESRD Network Assistance with:
|
ESRD Network Directory:
Contact your ESRD Network directly. Use the link below if you need assistance finding your ESRD Network’s contact information. |
ESRD Data Submission Deadlines for CY 2024 Data
Dialysis facilities must submit ESRD data by the deadlines listed below. Not submitting the required data by these deadlines puts your facility at risk for an ESRD QIP payment reduction. Please note that if the last day of the month falls on a Saturday, Sunday, or federal holiday, the deadline will occur on the next federal business day. The data submission deadlines apply to all collection types (Hemodialysis and Peritoneal Dialysis) and to all submission methods. CMS strongly recommends that facilities complete large data submissions and audit batch submitted data prior to data submission deadlines.
| EQRS Submission Schedule for: EQRS Clinical Data |
|
| Reporting Month | Data Submission Deadline |
| January 2024 | April 1, 2024, at 11:59 p.m. Pacific Time (PT) |
| February 2024 | April 30, 2024, at 11:59 p.m. PT |
| March 2024 | May 31, 2024, at 11:59 p.m. PT |
| April 2024 | July 1, 2024, at 11:59 p.m. PT |
| May 2024 | July 31, 2024, at 11:59 p.m. PT |
| June 2024 | September 3, 2024, at 11:59 p.m. PT |
| July 2024 | September 30, 2024, at 11:59 p.m. PT |
| August 2024 | October 31, 2024, at 11:59 p.m. PT |
| September 2024 | December 2, 2024, at 11:59 p.m. PT |
| October 2024 | December 31, 2024, at 11:59 p.m. PT |
| November 2024 | January 31, 2025, at 11:59 p.m. PT |
| December 2024 | February 28, 2025, at 11:59 p.m. PT |
| EQRS Submission Schedule for: Clinical Depression Screening and Follow Up Assessments |
|
| Assessment Period | Data Submission Deadline |
| January 2024 – December 2024 | February 28, 2025, at 11:59 PM PT |
| EQRS Submission Schedule for: ICH CAHPS Facility Attestation |
|
| Attestation Period | Submission Deadline |
| January 2024 – December 2024 | February 28, 2025, at 11:59 PM PT |
| EQRS Submission Schedule for: Commitment to Health Equity Attestation |
|
| Attestation Period | Submission Deadline |
| January 2024 – December 2024 | February 28, 2025, at 11:59 PM PT |
| NHSN Data Submission Deadlines for 2024 ESRD Data (BSI in Hemodialysis Patients, Dialysis Event Reporting, and COVID-19 Vaccination Coverage Among HCP) |
||
| Quarter | 2024 Reporting Months | Data Submission Deadline |
| 1 | January-March | July 1, 2024, at 11:59 p.m. PT |
| 2 | April-June | September 30, 2024, at 11:59 p.m. PT |
| 3 | July-September | December 31, 2024, at 11:59 p.m. PT |
| 4 | October-December | March 31, 2025, at 11:59 p.m. PT |
The information included as part of this newsletter is current as of the date of release.

